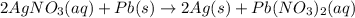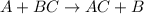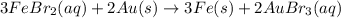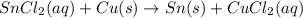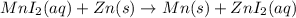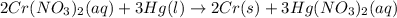Chemistry, 30.03.2020 23:13 jadajordan1010
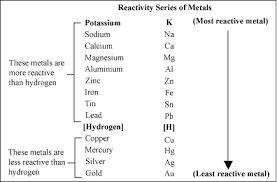
Based on the activity series, which one of the reactions below will occur? Based on the activity series, which one of the reactions below will occur? 3FeBr2 (aq) + 2Au (s) → 3Fe (s) + 2AuBr3 (aq) SnCl2 (aq) + Cu (s) → Sn (s) + CuCl2 (aq) Zn (s) + MnI2 (aq) → ZnI2 (aq) + Mn (s) 2AgNO3 (aq) + Pb (s) → 2Ag (s) + Pb(NO3)2 (aq) 3Hg (l) + 2Cr(NO3)3 (aq) → 3Hg(NO3)2 + 2Cr (s)

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
You know the right answer?
Based on the activity series, which one of the reactions below will occur? Based on the activity ser...
Questions



Chemistry, 15.06.2021 01:30


Mathematics, 15.06.2021 01:30

Social Studies, 15.06.2021 01:30

Mathematics, 15.06.2021 01:30


Mathematics, 15.06.2021 01:30


Chemistry, 15.06.2021 01:30

Chemistry, 15.06.2021 01:30


Mathematics, 15.06.2021 01:30

Chemistry, 15.06.2021 01:30

Mathematics, 15.06.2021 01:30

Geography, 15.06.2021 01:30



Mathematics, 15.06.2021 01:30