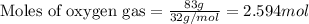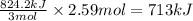Chemistry, 30.03.2020 23:14 constipatedcow18
Consider the reaction:
2Fe2O3⟶4Fe+3O2 ΔH∘rxn=+824.2 kJ
The formation of 83.0 g of O2 results in:
A. the absorption of 22800 kJ of heat.
B. the release of 2140 kJ of heat.
C. the absorption of 2140 kJ of heat.
D. the release of 713 kJ of heat.
E. the absorption of 713 kJ of heat.
F. the release of 22800 kJ of heat.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 23.06.2019 10:30
4al + 3o2 → 2al2o3 what does the "3" in front of o2 stand for? a) it indicates that there are 5 oxygen atoms after you add the coefficient and the subscript. b) it indicates that that there are are total of 6 oxygen atoms all bonded together as a single molecule. c) it indicates that there are 3 oxygen molecules chemically bonded to each other in the reaction. d) it indicates that there are 3 separate oxygen molecules in the reaction.
Answers: 2

Chemistry, 23.06.2019 14:00
How does electronegativity changes as we move from left to right across a period
Answers: 1
You know the right answer?
Consider the reaction:
2Fe2O3⟶4Fe+3O2 ΔH∘rxn=+824.2 kJ
The formation of 83.0 g of...
2Fe2O3⟶4Fe+3O2 ΔH∘rxn=+824.2 kJ
The formation of 83.0 g of...
Questions

Mathematics, 07.11.2020 04:30

Mathematics, 07.11.2020 04:30


History, 07.11.2020 04:30

Spanish, 07.11.2020 04:30

Biology, 07.11.2020 04:30

Mathematics, 07.11.2020 04:30



Law, 07.11.2020 04:30

Mathematics, 07.11.2020 04:30


History, 07.11.2020 04:30




Mathematics, 07.11.2020 04:30

Mathematics, 07.11.2020 04:30


History, 07.11.2020 04:30