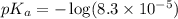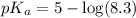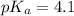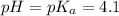The half‑equivalence point of a titration occurs half way to the equivalence point, where half of the analyte has reacted to form its conjugate, and the other half still remains unreacted. If 0.440 moles of a monoprotic weak acid ( K a = 8.3 × 10 − 5 ) is titrated with NaOH , what is the pH of the solution at the half‑equivalence point?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle
Answers: 3

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2


Chemistry, 22.06.2019 18:30
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2
You know the right answer?
The half‑equivalence point of a titration occurs half way to the equivalence point, where half of th...
Questions

Social Studies, 31.07.2021 01:20





Mathematics, 31.07.2021 01:20

Mathematics, 31.07.2021 01:20

Mathematics, 31.07.2021 01:20

Mathematics, 31.07.2021 01:20




English, 31.07.2021 01:20




Mathematics, 31.07.2021 01:20

Mathematics, 31.07.2021 01:20


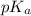 of weak acid.
of weak acid.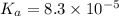
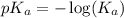
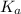 in this expression, we get:
in this expression, we get: