

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 23.06.2019 09:00
Avogradoa number was calculated by determining the number of atoms in?
Answers: 1

Chemistry, 23.06.2019 19:30
Which of the following is an example of ionic compound? a) sicl4 b) hcl c) cacl2 d) ccl4
Answers: 1
You know the right answer?
20.0 mL of a 0.80 M aqueous lead(II) nitrate solution is mixed with an excess of an aqueous sodium s...
Questions



History, 07.09.2019 03:10

Health, 07.09.2019 03:10

History, 07.09.2019 03:10

History, 07.09.2019 03:10

Mathematics, 07.09.2019 03:10

English, 07.09.2019 03:20

Mathematics, 07.09.2019 03:20


Mathematics, 07.09.2019 03:20

Mathematics, 07.09.2019 03:20

History, 07.09.2019 03:20







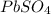 is 33%
is 33%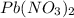 =
= 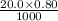 moles of
moles of 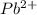
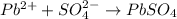
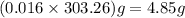
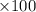 %
%  ) %
) % 


