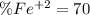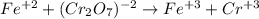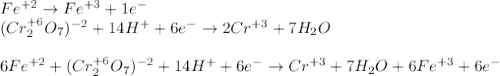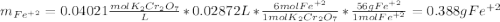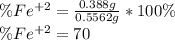Chemistry, 30.03.2020 23:08 GreenHerbz206
An iron ore sample weighing 0.5562 g is dissolved HCl (aq), and the iron is obtained as Fe2 in solution. This solution is then titrated with 28.72 mL of 0.04021 M K2Cr2O7 (aq). What is the percent by mass iron in the ore sample

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

You know the right answer?
An iron ore sample weighing 0.5562 g is dissolved HCl (aq), and the iron is obtained as Fe2 in solut...
Questions

Mathematics, 09.12.2019 22:31

Engineering, 09.12.2019 22:31


History, 09.12.2019 22:31

Mathematics, 09.12.2019 22:31

History, 09.12.2019 22:31


Mathematics, 09.12.2019 22:31



English, 09.12.2019 22:31

Mathematics, 09.12.2019 22:31

Chemistry, 09.12.2019 22:31

Mathematics, 09.12.2019 22:31


Physics, 09.12.2019 22:31

Biology, 09.12.2019 22:31

Mathematics, 09.12.2019 22:31

Mathematics, 09.12.2019 22:31

Geography, 09.12.2019 22:31