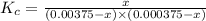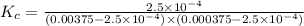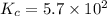3.0 mL of 0.02 M Fe(NO3)3 solution is mixed with 3.0 mL of 0.002 M NaNCS and diluted to the mark with HNO3 in 10 mL volumetric flask. The blood-red [Fe(NCS)]2+ ion that forms has an equilibrium molar concentration of 2.5*10-4 mol/L as determined from the calibration plot. Calculate the Kc for [Fe(NCS)]2+ formation. Assume the volumes are additive.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3


Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3
You know the right answer?
3.0 mL of 0.02 M Fe(NO3)3 solution is mixed with 3.0 mL of 0.002 M NaNCS and diluted to the mark wit...
Questions



Spanish, 04.05.2021 22:50

Health, 04.05.2021 22:50


Chemistry, 04.05.2021 22:50



Mathematics, 04.05.2021 22:50


Mathematics, 04.05.2021 22:50

Mathematics, 04.05.2021 22:50

Chemistry, 04.05.2021 22:50



Mathematics, 04.05.2021 22:50

Mathematics, 04.05.2021 22:50

English, 04.05.2021 22:50


Mathematics, 04.05.2021 22:50

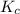 for
for ![[Fe(NCS)]^{2+}](/tpl/images/0571/7802/c2420.png) formation is
formation is  .
.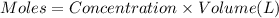
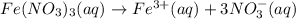
![[Fe(NO_3)_3]=0.02 M=[Fe^{3+}]](/tpl/images/0571/7802/da55e.png)
![[Fe^{3+}]=0.02 M](/tpl/images/0571/7802/a8178.png)
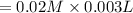
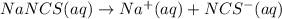
![[NaNCS]=0.002 M=[NCS^-]](/tpl/images/0571/7802/ed7be.png)
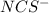 ion =
ion = ![[NCS^{-}]=0.002 M](/tpl/images/0571/7802/84149.png)
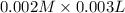
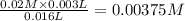
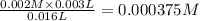
![Fe^{3+}+NCS^-\rightleftharpoons [Fe(NCS)]^{2+}](/tpl/images/0571/7802/1aeb7.png)
![[Fe(NCS)]^{2+}=x=2.5\times 10^{-4} M](/tpl/images/0571/7802/1a7a9.png)
![K_c=\frac{[[Fe(NCS)]^{2+}]}{[Fe^{3+}][NCS^-]}](/tpl/images/0571/7802/4616c.png)