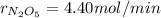Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2
You know the right answer?
The decomposition of dinitrogen pentoxide is described by the chemical equation 2 N2O5(g) → 4 NO2(g)...
Questions

English, 07.11.2020 08:10


Mathematics, 07.11.2020 08:10


Mathematics, 07.11.2020 08:10

Geography, 07.11.2020 08:10




Mathematics, 07.11.2020 08:10

Mathematics, 07.11.2020 08:10


Biology, 07.11.2020 08:10



Spanish, 07.11.2020 08:10



Mathematics, 07.11.2020 08:10

Mathematics, 07.11.2020 08:10