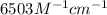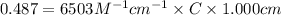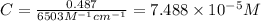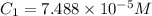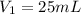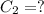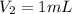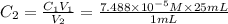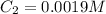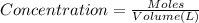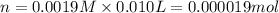An unknown amount of a compound with a molecular mass of 264.37 g/mol is dissolved in a 10 mL volumetric flask. A 1.00 mL aliquot of this solution is transferred to a 25 mL volumetric flask, and enough water is added to dilute to the mark. The absorbance of this diluted solution at 327 nm is 0.487 in a 1.000 cm cuvette. The molar absorptivity for this compound at 327 nm is ϵ 327 = 6503 M^(−1) cm^(−1).
(a) What is the concentration of the compound in the cuvette?
(b) What is the concentration of the compound in the 10-mL flask?
(c) How many milligrams of the compound were used to make the 10-mL solution?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 23.06.2019 13:20
Use the periodic table to answer the following questions. what is the predicted order of first ionization energies from highest to lowest for beryllium, calcium, magnesium, and strontium? o be > ca > mg > sr o be > mg > ca > sr o ca > sr> be > mg o sr > ca > mg > be done
Answers: 1

Chemistry, 23.06.2019 14:00
If the molar mass of the compound is 96.69 g/mol, what is the molecular formula of the compound?
Answers: 1
You know the right answer?
An unknown amount of a compound with a molecular mass of 264.37 g/mol is dissolved in a 10 mL volume...
Questions




Mathematics, 06.03.2021 08:30

Mathematics, 06.03.2021 08:30

Physics, 06.03.2021 08:30

Advanced Placement (AP), 06.03.2021 08:30


Chemistry, 06.03.2021 08:30



Mathematics, 06.03.2021 08:30


Mathematics, 06.03.2021 08:30




Biology, 06.03.2021 08:30

History, 06.03.2021 08:30

Mathematics, 06.03.2021 08:30

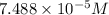 is the concentration of the compound in the cuvette.
is the concentration of the compound in the cuvette.
 = molar absorptivity of this solution =
= molar absorptivity of this solution =