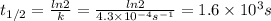Chemistry, 30.03.2020 21:50 karenjjensen
The rate constant, k, for a first-order reaction is equal to 4.3 × 10-4 s-1. What is the half-life for the reaction? The rate constant, k, for a first-order reaction is equal to 4.3 × 10-4 s-1. What is the half-life for the reaction? 1.9 × 103 s 1.2 × 103 s 1.6 × 103 s 3.0 × 10-4 s

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 23.06.2019 03:30
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3
You know the right answer?
The rate constant, k, for a first-order reaction is equal to 4.3 × 10-4 s-1. What is the half-life f...
Questions

English, 25.11.2020 02:00

Mathematics, 25.11.2020 02:00

English, 25.11.2020 02:00


Mathematics, 25.11.2020 02:00

Health, 25.11.2020 02:00




Mathematics, 25.11.2020 02:00



Mathematics, 25.11.2020 02:00


Mathematics, 25.11.2020 02:00

Mathematics, 25.11.2020 02:00

Computers and Technology, 25.11.2020 02:00

Social Studies, 25.11.2020 02:00

Biology, 25.11.2020 02:00

Spanish, 25.11.2020 02:00

![rate=k \times [A]^{m}](/tpl/images/0571/4200/f39c7.png)