
Chemistry, 30.03.2020 21:03 kharmaculpepper
When coal is burned, the sulfur present in coal is converted to sulfur dioxide (SO2), which is responsible for the acid rain phenomenon. S(s) + O2(g) → SO2(g) If 4.10 kg of S are reacted with oxygen, calculate the volume of SO2 gas (in mL) formed at 30°C and 1.16 atm.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is lincoln's purpose in writing this speech? question 1 options: to stress the difficulties of war to honor those who died in the war to call for an end to the war to call the country to join a new war
Answers: 1

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1
You know the right answer?
When coal is burned, the sulfur present in coal is converted to sulfur dioxide (SO2), which is respo...
Questions

Mathematics, 22.09.2019 06:30


Computers and Technology, 22.09.2019 06:30


Mathematics, 22.09.2019 06:30

Biology, 22.09.2019 06:30



Mathematics, 22.09.2019 06:30


Chemistry, 22.09.2019 06:30




Social Studies, 22.09.2019 06:30

English, 22.09.2019 06:30

Mathematics, 22.09.2019 06:30

History, 22.09.2019 06:30

Mathematics, 22.09.2019 06:30

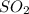 gas is,
gas is, 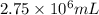
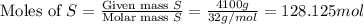
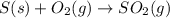
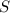 react to give 1 mole of
react to give 1 mole of 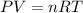
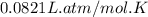
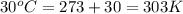
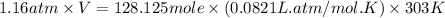
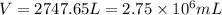 (1 L = 1000 mL)
(1 L = 1000 mL)

