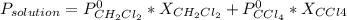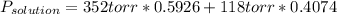Chemistry, 30.03.2020 21:07 villarrealc1987
The US Food and Drug Administration lists dichloromethane (CH2Cl2) and carbon tetrachloride (CCl4) among the many cancer causing volatile chlorinated organic compounds. An autoshop you are employed at keeps a jar (closed) of this solution to use as a solvent for cleaning parts. If the jar contains 1.60mol of dichloromethane and 1.10mol of CCl4, what would be the total pressure in the jar if the shop is kept at a consistent 23.5C

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
The US Food and Drug Administration lists dichloromethane (CH2Cl2) and carbon tetrachloride (CCl4) a...
Questions





History, 23.06.2019 07:30






Mathematics, 23.06.2019 07:30


Health, 23.06.2019 07:30




Mathematics, 23.06.2019 07:30


Mathematics, 23.06.2019 07:30

Social Studies, 23.06.2019 07:30