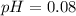Chemistry, 30.03.2020 21:10 Franklyn3220
A solution is made by adding 0.380 gg Ca(OH)2(s)Ca(OH)2(s), 50.0 mLmL of 1.45 MM HNO3HNO3, and enough water to make a final volume of 75.0 mLmL. Part A Assuming that all of the solid dissolves, what is the pH of the final solution

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2
You know the right answer?
A solution is made by adding 0.380 gg Ca(OH)2(s)Ca(OH)2(s), 50.0 mLmL of 1.45 MM HNO3HNO3, and enoug...
Questions

English, 06.05.2020 00:39










Mathematics, 06.05.2020 00:39

Biology, 06.05.2020 00:39


English, 06.05.2020 00:39





History, 06.05.2020 00:39

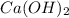 =
=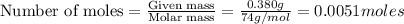
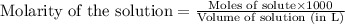
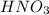 solution = 1.45 M
solution = 1.45 M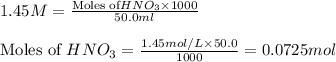
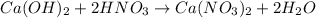
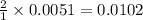 moles of
moles of 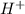 are left in 75.0 ml of solution
are left in 75.0 ml of solution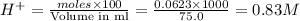
![pH=-\log [H^+]](/tpl/images/0571/2204/37e81.png)
![pH=-\log[0.83]](/tpl/images/0571/2204/f1115.png)