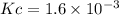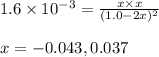Chemistry, 30.03.2020 21:15 4804102262
HI decomposes to H2 and I2 by the following equation: 2HI(g) → H2(g) + I2(g);Kc = 1.6 × 10−3 at 25∘C If 1.0 M HI is placed into a closed container and the reaction is allowed to reach equilibrium at 25∘C, what is the equilibrium concentration of H2 (g)?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

You know the right answer?
HI decomposes to H2 and I2 by the following equation: 2HI(g) → H2(g) + I2(g);Kc = 1.6 × 10−3 at 25∘C...
Questions

History, 21.03.2020 00:37



History, 21.03.2020 00:37


Mathematics, 21.03.2020 00:37





Mathematics, 21.03.2020 00:37

Mathematics, 21.03.2020 00:37






Chemistry, 21.03.2020 00:37


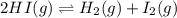
 for above equation follows:
for above equation follows:![K_c=\frac{[H_2][I_2]}{[HI]^2}](/tpl/images/0571/2457/ef85e.png)