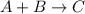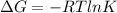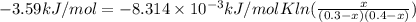Chemistry, 30.03.2020 20:50 Elepeodowke
Has a standard free‑energy change of − 3.59 kJ / mol at 25 °C. What are the concentrations of A , B , and C at equilibrium if, at the beginning of the reaction, their concentrations are 0.30 M, 0.40 M, and 0 M, respectively?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 23.06.2019 02:30
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1
You know the right answer?
Has a standard free‑energy change of − 3.59 kJ / mol at 25 °C. What are the concentrations of A , B...
Questions





Mathematics, 16.03.2020 00:49

Mathematics, 16.03.2020 00:49




Mathematics, 16.03.2020 00:50

Mathematics, 16.03.2020 00:50

Mathematics, 16.03.2020 00:50






Biology, 16.03.2020 00:52


Mathematics, 16.03.2020 00:52