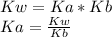Chemistry, 30.03.2020 20:27 viktoria1198zz
If you know Kb for ammonia, NH3, you can calculate the equilibrium constant, Ka, for the following reaction: NH4+ NH3 + H+ by the equation: Ka = 1 / Kb Ka = Kb / Kw Ka = Kw / Kb Ka = Kw × Kb None of these choices are correct.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3
You know the right answer?
If you know Kb for ammonia, NH3, you can calculate the equilibrium constant, Ka, for the following r...
Questions


History, 08.12.2021 05:50



Mathematics, 08.12.2021 05:50

Mathematics, 08.12.2021 05:50

English, 08.12.2021 05:50

Spanish, 08.12.2021 05:50

Mathematics, 08.12.2021 05:50

Social Studies, 08.12.2021 05:50

Mathematics, 08.12.2021 05:50



Spanish, 08.12.2021 05:50

History, 08.12.2021 05:50


Mathematics, 08.12.2021 05:50

English, 08.12.2021 05:50


Mathematics, 08.12.2021 05:50