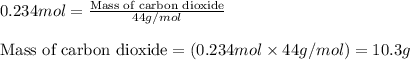Chemistry, 30.03.2020 20:39 lexhorton2002
Liquid hexane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 3.4 g of hexane is mixed with 22.6 g of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Round your answer to significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1
You know the right answer?
Liquid hexane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . S...
Questions

Mathematics, 16.10.2020 17:01

Biology, 16.10.2020 17:01


Mathematics, 16.10.2020 17:01


Mathematics, 16.10.2020 17:01

English, 16.10.2020 17:01


Mathematics, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01

Spanish, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01

Social Studies, 16.10.2020 17:01



Mathematics, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01

English, 16.10.2020 17:01

History, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01

 .....(1)
.....(1)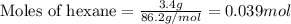
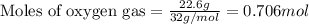
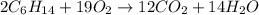
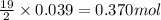 of oxygen gas
of oxygen gas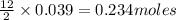 of carbon dioxide
of carbon dioxide