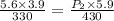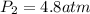Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 23.06.2019 01:30
In what way do investigations build scientific knowledge? the results of investigations lead to questions that cannot be tested. they reflect the opinions and social values of scientists, ensuring valid information. the results of investigations lead to new questions, which lead to new investigations. they are not influenced by the research of earlier scientists, so they are able to address gaps in understanding.i
Answers: 1

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1
You know the right answer?
A 3.9-L volume of ideal neon gas (monatomic) is at a pressure of 5.6 atm and a temperature of The at...
Questions

Mathematics, 01.09.2021 07:10

Mathematics, 01.09.2021 07:10

Computers and Technology, 01.09.2021 07:10

English, 01.09.2021 07:10


Social Studies, 01.09.2021 07:10

Mathematics, 01.09.2021 07:10

Mathematics, 01.09.2021 07:10


Mathematics, 01.09.2021 07:10

Mathematics, 01.09.2021 07:10

Arts, 01.09.2021 07:10



Mathematics, 01.09.2021 07:10




Mathematics, 01.09.2021 07:10

English, 01.09.2021 07:10

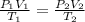
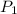 = initial pressure of gas = 5.6 atm
= initial pressure of gas = 5.6 atm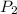 = final pressure of gas = ?
= final pressure of gas = ?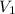 = initial volume of gas = 3.9 L
= initial volume of gas = 3.9 L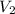 = final volume of gas = 5.9 L
= final volume of gas = 5.9 L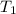 = initial temperature of gas = 330 K
= initial temperature of gas = 330 K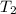 = final temperature of gas = 430 K
= final temperature of gas = 430 K