Chemistry, 30.03.2020 22:16 janahiac09
Part B When carbon is burned in air, it reacts with oxygen to form carbon dioxide. When 22.8 g of carbon were burned in the presence of 73.8 g of oxygen, 13.0 g of oxygen remained unreacted. What mass of carbon dioxide was produced? Express your answer to one decimal place and include the appropriate units. View Available Hint(s)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2



Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
Part B When carbon is burned in air, it reacts with oxygen to form carbon dioxide. When 22.8 g of ca...
Questions


Mathematics, 19.11.2020 19:10


English, 19.11.2020 19:10


World Languages, 19.11.2020 19:10





Mathematics, 19.11.2020 19:10


Arts, 19.11.2020 19:10


Biology, 19.11.2020 19:10

Mathematics, 19.11.2020 19:10

Mathematics, 19.11.2020 19:10

Advanced Placement (AP), 19.11.2020 19:10

History, 19.11.2020 19:10

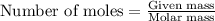 .....(1)
.....(1)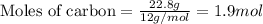
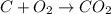
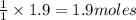 of carbon dioxide gas
of carbon dioxide gas