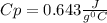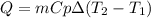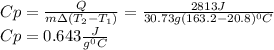Chemistry, 30.03.2020 22:18 Hockeypro1127
A 30.73 g 30.73 g sample of a substance is initially at 20.8 ° C. 20.8 °C. After absorbing 2813 J 2813 J of heat, the temperature of the substance is 163.2 ° C. 163.2 °C. What is the specific heat ( c c ) of the substance?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
A 30.73 g 30.73 g sample of a substance is initially at 20.8 ° C. 20.8 °C. After absorbing 2813 J 28...
Questions


Mathematics, 29.03.2021 04:50



English, 29.03.2021 04:50



Mathematics, 29.03.2021 04:50


Mathematics, 29.03.2021 04:50

English, 29.03.2021 04:50

Social Studies, 29.03.2021 04:50


History, 29.03.2021 04:50


Mathematics, 29.03.2021 04:50

Social Studies, 29.03.2021 04:50

Mathematics, 29.03.2021 04:50