
Chemistry, 30.03.2020 20:20 preciadogabriel40
Find the pH during the titration of 20.00 mL of 0.1000 M butanoic acid, CH3CH2CH2COOH (K a = 1.54 × 10 − 5), with 0.1000 M NaOH solution after the following additions of titrant (total volume of added base given):

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1


Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2
You know the right answer?
Find the pH during the titration of 20.00 mL of 0.1000 M butanoic acid, CH3CH2CH2COOH (K a = 1.54 ×...
Questions

Computers and Technology, 18.09.2019 16:10





Mathematics, 18.09.2019 16:10

English, 18.09.2019 16:10

Biology, 18.09.2019 16:10


English, 18.09.2019 16:10

History, 18.09.2019 16:10


Mathematics, 18.09.2019 16:10

Mathematics, 18.09.2019 16:10

Mathematics, 18.09.2019 16:10


Biology, 18.09.2019 16:10



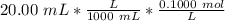
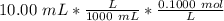
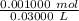
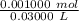
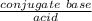
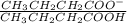
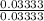
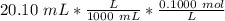
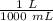


![[H_3O^+] = \frac{K \omega }{[OH^-]}](/tpl/images/0570/9707/cb9be.png)
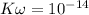
![[H_3O^+] = \frac{1.0*10^{-14}}{2.494*10^{-14}}](/tpl/images/0570/9707/791bd.png)

![[H_3O^+}]](/tpl/images/0570/9707/f6691.png)
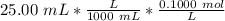
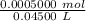
![[H_3O^+] = \frac{K \omega }{[OH^+]}](/tpl/images/0570/9707/32cc9.png)
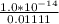
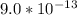 M
M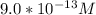 ]
]

