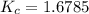Chemistry, 30.03.2020 19:34 jallison61
At a certain temperature, 4.0 mol NH3 is introduced into a 2.0 L container, and the NH3 partially dissociates by the reaction below. 2 NH3(g) equilibrium reaction arrow N2(g) 3 H2(g) At equilibrium, 2.0 mol NH3 remains. What is the value of K for this reaction

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 23.06.2019 02:00
The bone of a dinosaur and the imprint of a leaf are examples of which kind of fossils? a) index b) body c) amber d) trace
Answers: 1
You know the right answer?
At a certain temperature, 4.0 mol NH3 is introduced into a 2.0 L container, and the NH3 partially di...
Questions


Mathematics, 22.08.2019 22:30



Business, 22.08.2019 22:30


Mathematics, 22.08.2019 22:30









Computers and Technology, 22.08.2019 22:30




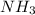 = 4.0 mole
= 4.0 mole
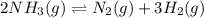
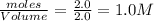
![K_c=\frac{[x]\times [3x]^3}{[(2-2x)]^2}](/tpl/images/0570/9112/70e97.png)
![K_c=\frac{[0.5]\times [3\times 0.5]^3}{[(2-2\times 0.5)]^2}](/tpl/images/0570/9112/97489.png)