
Chemistry, 30.03.2020 19:35 kaliemn7oyqxuy
A major component of gasoline is octane (C8H18). When liquid octane is burned in the air it reacts with oxygen gas to produce carbon dioxide gas and water vapor.
Calculate the moles of carbon dioxide produced by the reaction of 0.085 mol of oxygen. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3
You know the right answer?
A major component of gasoline is octane (C8H18). When liquid octane is burned in the air it reacts w...
Questions



Mathematics, 10.05.2021 19:50

Chemistry, 10.05.2021 19:50



Mathematics, 10.05.2021 19:50


History, 10.05.2021 19:50

Mathematics, 10.05.2021 19:50

Health, 10.05.2021 19:50

Social Studies, 10.05.2021 20:00





Mathematics, 10.05.2021 20:00

Mathematics, 10.05.2021 20:00


Computers and Technology, 10.05.2021 20:00

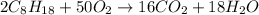
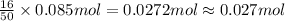 of carbon dioxide
of carbon dioxide 

