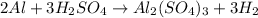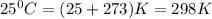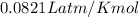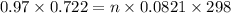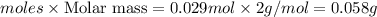G A sample that contains aluminum is reacted with sulfuric acid according to the equation given below. When the aluminum dissolved the total volume of gas collected over water at 25 °C is 0.722 L at a total pressure of 739 mm Hg. What mass of hydrogen gas is collected?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
As a part of an experiment a student burns propane to produce carbon dioxide and water she remembers that she must follow the law conservation of matter when writing a balanced chemical equation which of these equation adheres to the law of conservation of matter
Answers: 1

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 02:40
For a patient with the following pes statement and interventions, which would be the most appropriate monitoring and evaluating data? pes statement: inadequate calcium intake related to food and nutrition related knowledge deficit as evidenced by statements that the only dietary source of calcium is milk and she believes that she is lactose intolerant. patient’s nutrition prescription is for a diet providing 1200 mg calcium per day. patient was provided with in-depth nutrition education on alternative dietary and supplement sources of calcium. a. calcium intake (at subsequent visit) b. knowledge assessment by asking patient to identify food sources from menus and shopping list (at the end of the current visit) c. serum calcium (at next visit) d. both a and b e. both a and c
Answers: 2

You know the right answer?
G A sample that contains aluminum is reacted with sulfuric acid according to the equation given belo...
Questions




Arts, 21.04.2020 04:41


Mathematics, 21.04.2020 04:41

Geography, 21.04.2020 04:41

Computers and Technology, 21.04.2020 04:41





Social Studies, 21.04.2020 04:41

Mathematics, 21.04.2020 04:41

History, 21.04.2020 04:41

Social Studies, 21.04.2020 04:41




Mathematics, 21.04.2020 04:41