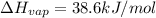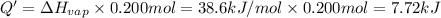Chemistry, 30.03.2020 21:38 angiejtc0908
G Ethanol boils at 78.4 °C with \DeltaΔHvap = 38.6 kJ/mol. A 0.200-mol sample of ethanol is heated from some colder temperature up to 78.4 °C, which requires 1.05 kJ of heat, and then vaporized. What will be the total amount of heat required (for both the heating and the vaporizing)?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
You know the right answer?
G Ethanol boils at 78.4 °C with \DeltaΔHvap = 38.6 kJ/mol. A 0.200-mol sample of ethanol is heated f...
Questions

Mathematics, 18.12.2020 18:00

Mathematics, 18.12.2020 18:00


Arts, 18.12.2020 18:00

Chemistry, 18.12.2020 18:00



Physics, 18.12.2020 18:00


Mathematics, 18.12.2020 18:00

Mathematics, 18.12.2020 18:00



Law, 18.12.2020 18:00


Mathematics, 18.12.2020 18:00



Chemistry, 18.12.2020 18:00