

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 02:00
What are fossils of organisms that existed over a wide area but only for a limited time period called?
Answers: 2
You know the right answer?
A solution containing CaCl2 is mixed with a solution of Li2C2O4 to form a solution that is 3.5 × 10-...
Questions

Mathematics, 01.04.2021 07:30


English, 01.04.2021 07:30


Biology, 01.04.2021 07:30


History, 01.04.2021 07:30


Mathematics, 01.04.2021 07:30

Chemistry, 01.04.2021 07:30

Mathematics, 01.04.2021 07:30


Geography, 01.04.2021 07:40




Business, 01.04.2021 07:40

Mathematics, 01.04.2021 07:40

Mathematics, 01.04.2021 07:40

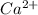 =
= 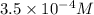
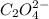 =
= 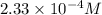
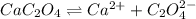
![K_{sp}=[Ca^{2+}][C_2O_4^{2-}]=2.3\times 10^{-9}](/tpl/images/0570/7450/df299.png)
![Q_{sp}=[Ca^{2+}][C_2O_4^{2-}]](/tpl/images/0570/7450/af00c.png)
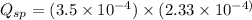
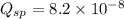
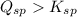 that means a white solid precipitate of calcium oxalate will be formed when the solutions are mixed.
that means a white solid precipitate of calcium oxalate will be formed when the solutions are mixed.


