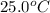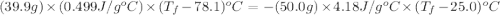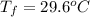A hot lump of 39.9 g of iron at an initial temperature of 78.1 °C is placed in 50.0 mL H 2 O initially at 25.0 °C and allowed to reach thermal equilibrium. What is the final temperature of the iron and water, given that the specific heat of iron is 0.449 J/(g⋅°C)? Assume no heat is lost to surroundings.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

You know the right answer?
A hot lump of 39.9 g of iron at an initial temperature of 78.1 °C is placed in 50.0 mL H 2 O initial...
Questions

Mathematics, 19.10.2021 16:10

Chemistry, 19.10.2021 16:10

Mathematics, 19.10.2021 16:10


Mathematics, 19.10.2021 16:10

History, 19.10.2021 16:10


History, 19.10.2021 16:10

Mathematics, 19.10.2021 16:10

Mathematics, 19.10.2021 16:10


Mathematics, 19.10.2021 16:10


English, 19.10.2021 16:10


Mathematics, 19.10.2021 16:10

Spanish, 19.10.2021 16:10


History, 19.10.2021 16:10

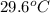
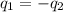
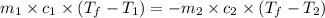
 = specific heat of iron =
= specific heat of iron = 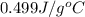
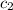 = specific heat of water =
= specific heat of water = 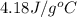
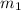 = mass of iron = 39.9 g
= mass of iron = 39.9 g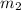 = mass of water =
= mass of water = 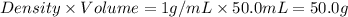
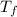 = final temperature of mixture = ?
= final temperature of mixture = ?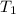 = initial temperature of iron =
= initial temperature of iron = 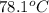
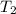 = initial temperature of water =
= initial temperature of water =