

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3
You know the right answer?
Suppose in an experiment to determine the amount of sodium hypochlorite in bleach, 0.0000524 mol K I...
Questions


Mathematics, 20.07.2020 21:01



Health, 20.07.2020 21:01

English, 20.07.2020 21:01




Mathematics, 20.07.2020 21:01




Mathematics, 20.07.2020 21:01





Biology, 20.07.2020 21:01

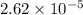 moles
moles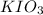 solution given = 0.0000524 moles
solution given = 0.0000524 moles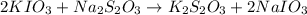
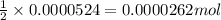 of sodium thiosulfate
of sodium thiosulfate


