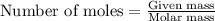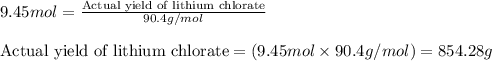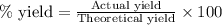Consider the balanced equation for the following reaction:
3Ca(ClO3)2(aq) + 2Li3PO4(aq) → Ca3(...

Chemistry, 30.03.2020 17:35 brazilmade1
Consider the balanced equation for the following reaction:
3Ca(ClO3)2(aq) + 2Li3PO4(aq) → Ca3(PO4)2(s) + 6LiClO3(aq)
Determine the theoretical yield of LiClO3(aq) in grams if the percent yield of LiClO3(aq) is 81.0% and 9.45 moles of LiClO3(aq) forms.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1
You know the right answer?
Questions









English, 28.08.2019 20:10





English, 28.08.2019 20:10



Physics, 28.08.2019 20:10