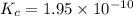Chemistry, 30.03.2020 17:28 natalie2sheffield
G Potassium chlorate decomposes to product potassium chloride and oxygen gas. 2KClO3(s) ⇔ 2KCl(s) + 3O2(g) When this reaction was run at room temperature, the following equilbrium concentrations were measured: [O2] = 0.0500 M; [KCl] = 0.00250 M; [KClO3] = 2.00 M What is the equilibrium constant for this reaction?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1



Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2
You know the right answer?
G Potassium chlorate decomposes to product potassium chloride and oxygen gas. 2KClO3(s) ⇔ 2KCl(s) +...
Questions




















English, 05.11.2019 01:31

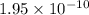 is the equilibrium constant for this reaction.
is the equilibrium constant for this reaction.![[O_2] = 0.0500 M](/tpl/images/0570/5147/94139.png)
![[KCl] = 0.00250 M](/tpl/images/0570/5147/3a302.png)
![[KClO_3] = 2.00 M](/tpl/images/0570/5147/90bf6.png)
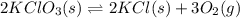
![K_c=\frac{[KCl]^2[O_2]^3}{[KClO_3]^2}](/tpl/images/0570/5147/bd160.png)
![=\frac{[0.00250 M]^2[0.0500 M]^3}{[2.00 M]^2}](/tpl/images/0570/5147/c5974.png)