
Chemistry, 30.03.2020 17:18 91miketaylor
Your lab group forgot to record the initial volume measurement on the buret and assumed later that it started at 0.00 mL when the actual starting buret reading was 2.00 mL. Would your final calculated molarity of the unknown acid be higher, lower or equal to the actual concentration

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2
You know the right answer?
Your lab group forgot to record the initial volume measurement on the buret and assumed later that i...
Questions


English, 29.09.2020 14:01


Mathematics, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01




Geography, 29.09.2020 14:01


Mathematics, 29.09.2020 14:01


Biology, 29.09.2020 14:01

Health, 29.09.2020 14:01



Mathematics, 29.09.2020 14:01



Mathematics, 29.09.2020 14:01

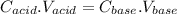
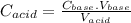
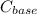 and
and 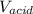 are constants.
are constants.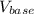 is lesser. Hence calculated concentration or molarity of acid is higher than the actual concentration.
is lesser. Hence calculated concentration or molarity of acid is higher than the actual concentration.

