
Aqueous sulfuric acid will react with solid sodium hydroxide to produce aqueous sodium sulfate and liquid water . Suppose 0.98 g of sulfuric acid is mixed with 0.240 g of sodium hydroxide. Calculate the maximum mass of sodium sulfate that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1

Chemistry, 23.06.2019 18:30
The first step in creating a budget is a) getting a bank account. b) cutting back on expenses. c) knowing what your income is. d) listing all of your expenses.
Answers: 1
You know the right answer?
Aqueous sulfuric acid will react with solid sodium hydroxide to produce aqueous sodium sulfate and l...
Questions



English, 29.01.2021 19:30

Mathematics, 29.01.2021 19:30


Mathematics, 29.01.2021 19:30

English, 29.01.2021 19:30



Biology, 29.01.2021 19:30

Mathematics, 29.01.2021 19:30

Biology, 29.01.2021 19:30


Mathematics, 29.01.2021 19:30

Arts, 29.01.2021 19:30

Advanced Placement (AP), 29.01.2021 19:30


Mathematics, 29.01.2021 19:30


History, 29.01.2021 19:30

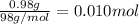

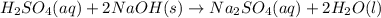
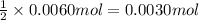 of sulfuric acid
of sulfuric acid

