Chemistry, 30.03.2020 17:02 Diamondnado3046
You mix 200. mL of 0.400M HCl with 200. mL of 0.400M NaOH in a coffee cup calorimeter. The temperature of the solution goes from 25.10°C to 27.78° C. What is the molar enthalpy of neutralization of the acid in kJ/mol? Assume all densities are 1.00 g/mL and the specific heat capacities are 4.184 J/g*K.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3


Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3
You know the right answer?
You mix 200. mL of 0.400M HCl with 200. mL of 0.400M NaOH in a coffee cup calorimeter. The temperatu...
Questions



Mathematics, 11.05.2021 15:30



Law, 11.05.2021 15:30

History, 11.05.2021 15:30



Spanish, 11.05.2021 15:30

Mathematics, 11.05.2021 15:30

Business, 11.05.2021 15:30


Mathematics, 11.05.2021 15:30



Mathematics, 11.05.2021 15:30

Mathematics, 11.05.2021 15:30


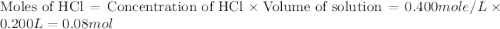
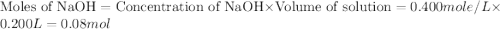

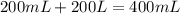
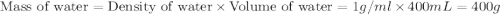
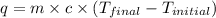
 = specific heat of water =
= specific heat of water = 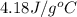
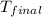 = final temperature of water =
= final temperature of water = 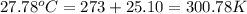
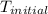 = initial temperature of metal =
= initial temperature of metal = 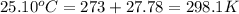
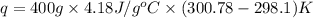
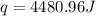
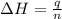
 = enthalpy of neutralization = ?
= enthalpy of neutralization = ?