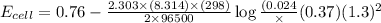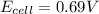In a test of a new reference electrode, a chemist constructs a voltaic cell consisting of a Zn/Zn2+ half-cell and an H2/H+ half-cell under the following conditions: [Zn2+ ] = 0.024 M [H+ ]= 1.3 M partial pressure of H2 = 0.37 atm. Calculate Ecell at 298 K (enter to 3 decimal places). Zn2+ (aq) + 2eLaTeX: -− LaTeX: \longrightarrow⟶ Zn(s) E° = LaTeX: -−0.76 V 2H+ (aq) + 2eLaTeX: -−LaTeX: \longrightarrow⟶ H2(g) E° = 0.00 V

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Monkeys and bats have similar bone structure in their forelimbs. however, monkeys have longer forelimbs to use for climbing and swinging in trees. bats have shorter forelimbs to use for flight. which term best describes how monkey and bat forelimbs are related to each other? a. homologous b. embryonic c. analogous d. vestigial
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1
You know the right answer?
In a test of a new reference electrode, a chemist constructs a voltaic cell consisting of a Zn/Zn2+...
Questions


Mathematics, 09.03.2020 00:49

History, 09.03.2020 00:50




Mathematics, 09.03.2020 00:50

Mathematics, 09.03.2020 00:51


Mathematics, 09.03.2020 00:51


History, 09.03.2020 00:51








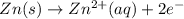
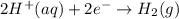
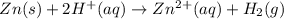
![E^o_{[Zn^{2+}/Zn]}=-0.76V](/tpl/images/0570/4548/c67da.png)
![E^o_{[H^+/H_2]}=0.00V](/tpl/images/0570/4548/4b48a.png)
![E^o=E^o_{[cathode]}-E^o_{[anode]}](/tpl/images/0570/4548/51f3e.png)
![E^o=E^o_{[H^+/H_2]}-E^o_{[Zn^{2+}/Zn]}](/tpl/images/0570/4548/91ffc.png)
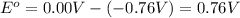
![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Zn^{2+}]\times (p_{H_2})}{[H^+]^2}](/tpl/images/0570/4548/307e3.png)
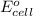 = electrode potential of the cell = ?
= electrode potential of the cell = ?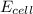 = emf of the cell = 0.76 V
= emf of the cell = 0.76 V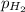 = 0.37 atm
= 0.37 atm![[Zn^{2+}]](/tpl/images/0570/4548/9c01a.png) = 0.024 M
= 0.024 M![[H^{+}]](/tpl/images/0570/4548/85507.png) = 1.3 M
= 1.3 M