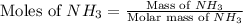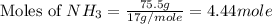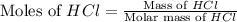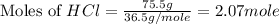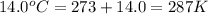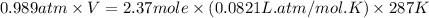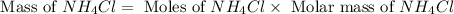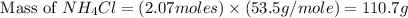Chemistry, 30.03.2020 17:04 vicinimlv19
What is the mass of the solid NH4Cl formed when 75.5 g of NH3 is mixed with an equal mass of HCl? What is the volume of the gas remaining, measured at 14.0°C and 752 mmHg? What gas is it? NH3(g) + HCl(g) → NH4Cl(s) What is the mass of the NH4Cl produced?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

You know the right answer?
What is the mass of the solid NH4Cl formed when 75.5 g of NH3 is mixed with an equal mass of HCl? Wh...
Questions


Health, 18.10.2020 16:01

English, 18.10.2020 16:01





Mathematics, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01

Social Studies, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01


Mathematics, 18.10.2020 16:01



Mathematics, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01

English, 18.10.2020 16:01

English, 18.10.2020 16:01

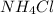 produced is, 110.7 grams.
produced is, 110.7 grams.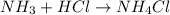
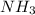 and HCl
and HCl