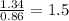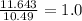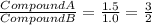Chemistry, 30.03.2020 16:16 kenishawilkinsoy4mgw
Sulfur reacts with oxygen and creates two compounds. Compound A contains 1.34 g of sulfur for every 0.86 g of oxygen. Compound B contains 11.63 g of sulfur for every 10.49 g of oxygen. What is the mass ratio of oxygen rounded to the nearest whole number

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 08:00
How many distinct monochlorinated products, including stereoisomers, can result when the alkane below is heated in the presence of cl2? 3 4 5 6 7?
Answers: 3

Chemistry, 23.06.2019 13:30
Which of the following is true regarding chemical and nuclear reactions?
Answers: 1

Chemistry, 23.06.2019 14:00
If the molar mass of the compound is 96.69 g/mol, what is the molecular formula of the compound?
Answers: 1

Chemistry, 23.06.2019 18:30
You form water vapor by mixing oxygen and hydrogen at 730°c in a 5.4-liter container. this is the equation for the reaction: o2(g) + 2h2(g) → 2h2o(g). the partial pressure of oxygen before the reaction is 122.3 kilopascals, and there is excess hydrogen. how many moles of water are formed?
Answers: 3
You know the right answer?
Sulfur reacts with oxygen and creates two compounds. Compound A contains 1.34 g of sulfur for every...
Questions

Mathematics, 14.06.2021 04:00

Mathematics, 14.06.2021 04:00

History, 14.06.2021 04:00



Mathematics, 14.06.2021 04:00






Mathematics, 14.06.2021 04:10



Mathematics, 14.06.2021 04:10




Mathematics, 14.06.2021 04:10

Business, 14.06.2021 04:10