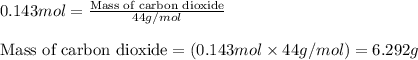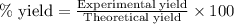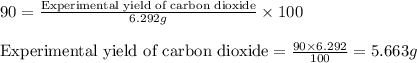Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
Consider the balanced equation for the following reaction:
7O2(g) + 2C2H6(g) → 4CO2(g) + 6H2O(...
7O2(g) + 2C2H6(g) → 4CO2(g) + 6H2O(...
Questions



Geography, 26.07.2019 09:30






Biology, 26.07.2019 09:30



Mathematics, 26.07.2019 09:30



Mathematics, 26.07.2019 09:30

Mathematics, 26.07.2019 09:30




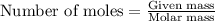 .....(1)
.....(1)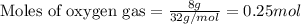
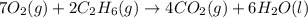
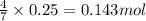 of carbon dioxide
of carbon dioxide