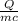Chemistry, 29.03.2020 19:47 jennainglish
30,000 J of heat are added to 23.0 kg of steel to reach a final temperature of 140
°C. What was the initial temperature of the steel? (csteel =490J/kg * °C)
1. First find AT using the formula q=mcAT
2. Rearrange to solve for initial temperature using AT=Tfinal -Tinital
I need help quickly

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:40
What type of solution is formed if 10 g of kclo3 are dissolved in 100g of water at 30
Answers: 2

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 23.06.2019 11:30
A) equal lines b) parallel lines c) perpendicular lines d) none of the above
Answers: 1

You know the right answer?
30,000 J of heat are added to 23.0 kg of steel to reach a final temperature of 140
°C. What wa...
°C. What wa...
Questions

Mathematics, 13.01.2020 21:31


Chemistry, 13.01.2020 21:31

Mathematics, 13.01.2020 21:31

Mathematics, 13.01.2020 21:31

History, 13.01.2020 21:31


Mathematics, 13.01.2020 21:31



Mathematics, 13.01.2020 21:31

English, 13.01.2020 21:31

Computers and Technology, 13.01.2020 21:31


Mathematics, 13.01.2020 21:31


Mathematics, 13.01.2020 21:31

French, 13.01.2020 21:31

Mathematics, 13.01.2020 21:31