Note the average atomic mass of copper on the nano-balance. Add atoms to the
larger balance un...

Note the average atomic mass of copper on the nano-balance. Add atoms to the
larger balance until it registers the same number in g) as the reading on the nano-balance
(in u). Use the Exponent slider to help get the correct amount. Stop adding atoms when the
readings on both balances matchi exactly to the nearest 0.001 y).
How many atoms did you need to add?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Theoretically, which metal should be the most reactive? hydrogen lithium francium fluorine
Answers: 1


Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
Questions

Mathematics, 11.10.2019 10:50


Mathematics, 11.10.2019 10:50

English, 11.10.2019 10:50

Mathematics, 11.10.2019 10:50

Mathematics, 11.10.2019 10:50


Mathematics, 11.10.2019 10:50


History, 11.10.2019 10:50


Mathematics, 11.10.2019 10:50

Mathematics, 11.10.2019 10:50

Social Studies, 11.10.2019 10:50


History, 11.10.2019 10:50

Mathematics, 11.10.2019 10:50

Mathematics, 11.10.2019 10:50


 1
1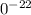 g
.
g
.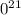 atoms.
atoms.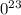 atoms or molecules of that substance - this is known as Avogadro's number.
atoms or molecules of that substance - this is known as Avogadro's number.

