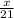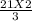Chemistry, 28.03.2020 22:17 NetherisIsTheQueen
Consider the following balanced reaction between hydrogen and nitrogen to form ammonia:
3H2(g)+N2(g)→2NH3(g)
How many moles of NH3 can be produced from 21.0 mol of H2 and excess N2?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3


Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3
You know the right answer?
Consider the following balanced reaction between hydrogen and nitrogen to form ammonia:
...
...
Questions


Biology, 14.12.2020 05:00





Physics, 14.12.2020 05:00

Mathematics, 14.12.2020 05:00


Mathematics, 14.12.2020 05:00

Social Studies, 14.12.2020 05:00

Chemistry, 14.12.2020 05:00

Arts, 14.12.2020 05:00

Mathematics, 14.12.2020 05:00

Arts, 14.12.2020 05:00

Social Studies, 14.12.2020 05:00

Biology, 14.12.2020 05:00

Mathematics, 14.12.2020 05:00

Mathematics, 14.12.2020 05:00

Mathematics, 14.12.2020 05:00

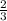 =
=