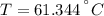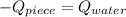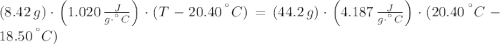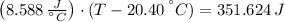Chemistry, 28.03.2020 18:38 veroushkarose7326
An 8.42 g piece of metal with a specific heat of 1.020 J g-1 k-1 wasn't heated to an unknown temperature. The metal was then placed in 44.2 g of water with an initial temperature of 18.50 C. If the final temperature of the water was 20.40 C, what temperature was the metal initially heated to (in C).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
An 8.42 g piece of metal with a specific heat of 1.020 J g-1 k-1 wasn't heated to an unknown tempera...
Questions


History, 28.07.2019 01:30





History, 28.07.2019 01:30



Social Studies, 28.07.2019 01:30


Mathematics, 28.07.2019 01:30

Social Studies, 28.07.2019 01:30

English, 28.07.2019 01:30

Mathematics, 28.07.2019 01:30





History, 28.07.2019 01:30