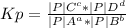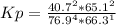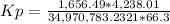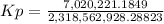Hydrogen chloride and oxygen react to form chlorine and water, like this:
4 HCl(g) + O2(g) → 2 Cl2(g) + 2 H2O(g)
Also, a chemist finds that at a certain temperature the equilibrium mixture of hydrogen chloride, oxygen, chlorine, and water has the following composition:
COMPOUND Pressure at equilibrium
HCl 76.9 atm
O2 66.3 atm
Cl2 40.7 atm
H2O 65.1 atm
Calculate the value of the equilibrium constant Kp for this reaction. Round your answer to 2 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
The length of a vector arrow represents its magnitude and the point represents its direction true or false apex
Answers: 3

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
Hydrogen chloride and oxygen react to form chlorine and water, like this:
4 HCl(g) + O2...
4 HCl(g) + O2...
Questions


English, 07.10.2021 14:40



Biology, 07.10.2021 14:40

Biology, 07.10.2021 14:40


Chemistry, 07.10.2021 14:40





Biology, 07.10.2021 14:40


Medicine, 07.10.2021 14:40



Social Studies, 07.10.2021 14:50

English, 07.10.2021 14:50