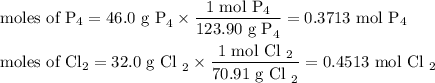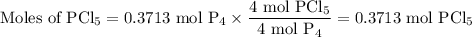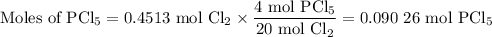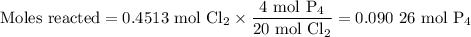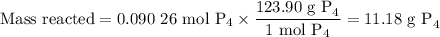Chemistry, 28.03.2020 04:02 shinyelish6
04.05 mol
The reaction of chlorine gas with solid phosphorus (P4) produces solid
phosphorus pentachloride. When 32.0 g of chlorine reacts with 46.0 g of P4.
What is the mass in excess of the excess reactantt?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

You know the right answer?
04.05 mol
The reaction of chlorine gas with solid phosphorus (P4) produces solid
phospho...
The reaction of chlorine gas with solid phosphorus (P4) produces solid
phospho...
Questions

Physics, 22.07.2019 04:30


History, 22.07.2019 04:30

Social Studies, 22.07.2019 04:30

Social Studies, 22.07.2019 04:30





English, 22.07.2019 04:30





Mathematics, 22.07.2019 04:30

Biology, 22.07.2019 04:30

English, 22.07.2019 04:30

English, 22.07.2019 04:30

Mathematics, 22.07.2019 04:30