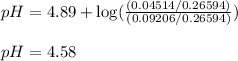Chemistry, 28.03.2020 04:02 cupcake20019peehui
An analytical chemist is titrating of a solution of propionic acid with a solution of 224.9 ml of a 0.6100M solution of propionic acid (HC2H5CO2) with a 1.1000M solution of KOH. The pKa of proionic acid 4.89.
Calculate the pH of the acid solution after the chemist has added 41.04mL of the KOH solution to it.
Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of solution added. Round your answer to decimal places.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3
You know the right answer?
An analytical chemist is titrating of a solution of propionic acid with a solution of 224.9 ml of a...
Questions

History, 13.01.2021 05:30






English, 13.01.2021 05:30



History, 13.01.2021 05:30

Mathematics, 13.01.2021 05:30


Chemistry, 13.01.2021 05:30

English, 13.01.2021 05:30

English, 13.01.2021 05:30

English, 13.01.2021 05:30




Mathematics, 13.01.2021 05:30

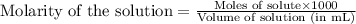 .....(1)
.....(1)
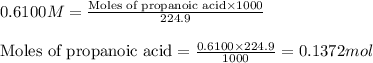
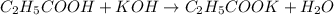
![pH=pK_a+\log(\frac{[\text{salt}]}{[acid]})](/tpl/images/0568/6689/3d096.png)
![pH=pK_a+\log(\frac{[C_2H_5COOK]}{[C_2H_5COOH]})](/tpl/images/0568/6689/3ede9.png)
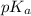 = negative logarithm of acid dissociation constant of propanoic acid = 4.89
= negative logarithm of acid dissociation constant of propanoic acid = 4.89![[C_2H_5COOK]=\frac{0.04514}{0.26594}](/tpl/images/0568/6689/11196.png)
![[C_2H_5COOH]=\frac{0.09206}{0.26594}](/tpl/images/0568/6689/86178.png)