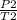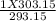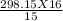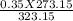Gay-Lussac Problems
P./T1 = P2/T2 - Remember, T is in KELVIN, add 273 to any temp given to you in °C to convert
1. Determine the pressure change when a constant volume of gas at 1.00 atm is heated from 20.0
°C to 30.0 °C.
2. If a gas in a closed container is pressurized from 15.0 atmospheres to 16.0 atmospheres and its
original temperature was 25.0 °C, what would the final temperature of the gas be?
3. A gas has a pressure of 0.35 atm at 50.0 °C. What is the pressure at standard temperature?
Boyle's law Drobleme

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
Working with si (metric) units for each of the following commonly used measurements, indicate its symbol. liter gram milliliter kilogram meter centigram milligram centimeter kilometer second millimeter milliseconds
Answers: 1

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1
You know the right answer?
Gay-Lussac Problems
P./T1 = P2/T2 - Remember, T is in KELVIN, add 273 to any temp given to you...
P./T1 = P2/T2 - Remember, T is in KELVIN, add 273 to any temp given to you...
Questions

Mathematics, 05.02.2020 01:47

Mathematics, 05.02.2020 01:47

History, 05.02.2020 01:47

Physics, 05.02.2020 01:47


Mathematics, 05.02.2020 01:47

Chemistry, 05.02.2020 01:47


Mathematics, 05.02.2020 01:47



Social Studies, 05.02.2020 01:47

Mathematics, 05.02.2020 01:47



Chemistry, 05.02.2020 01:47

Biology, 05.02.2020 01:47

Mathematics, 05.02.2020 01:47

History, 05.02.2020 01:47

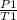 =
=