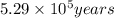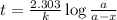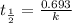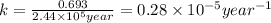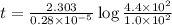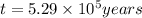Radioactive plutonium-239 (t½ = 2.44 x 105 yr) is used in nuclear reactors and atomic bombs. If there are 4.4 x 102 g of the isotope in a small atomic bomb, how long will it take (in yr) for the substance to decay to 1.0 x 102 g, too small an amount for an effective bomb? This radioactive decay follows first order kinetics.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2


Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3
You know the right answer?
Radioactive plutonium-239 (t½ = 2.44 x 105 yr) is used in nuclear reactors and atomic bombs. If ther...
Questions

Mathematics, 08.09.2021 19:30

Mathematics, 08.09.2021 19:30


Mathematics, 08.09.2021 19:30


Mathematics, 08.09.2021 19:30

Mathematics, 08.09.2021 19:30


Physics, 08.09.2021 19:30

History, 08.09.2021 19:30

Health, 08.09.2021 19:30


Mathematics, 08.09.2021 19:30




Mathematics, 08.09.2021 19:30


Mathematics, 08.09.2021 19:30

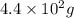 to
to  is
is