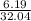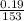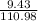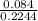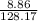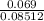Chemistry, 27.03.2020 19:31 carlosleblanc26
Calculate the molarity of each of the following solutions. (mol/L)
(a) 6.19 g of methanol (CH3OH) in 1.50 × 102 mL of solution:
mol/L
(b) 9.43 g of calcium chloride (CaCl2) in 2.20 × 102 mL of solution:
mol/L
(c) 8.86 g of naphthalene (C10H8) in 85.2 mL of benzene solution:
mol/L

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

You know the right answer?
Calculate the molarity of each of the following solutions. (mol/L)
(a) 6.19 g of methano...
(a) 6.19 g of methano...
Questions

Mathematics, 17.11.2019 23:31




Mathematics, 17.11.2019 23:31


Physics, 17.11.2019 23:31

History, 17.11.2019 23:31

History, 17.11.2019 23:31








Biology, 17.11.2019 23:31

Computers and Technology, 17.11.2019 23:31


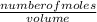 equation 1
equation 1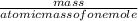 equation 2
equation 2