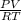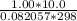Chemistry, 27.03.2020 18:26 ebookhardt917
A 4.00g sample of helium has a volume of 24.4L at a temperature of 25.0oC and a pressure of 1.00 atm. The volume of the helium is reduced to 10.4L, but the temperature and pressure of the gas are kept con- stant. What is the new quantity of the gas, in moles? Show all work using units and sig figs.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:30
How many miles of calcium oxide will be produced when 1.6 miles of iron (iii) oxide react with calcium phosphate
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2


Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
A 4.00g sample of helium has a volume of 24.4L at a temperature of 25.0oC and a pressure of 1.00 atm...
Questions





Mathematics, 19.05.2020 16:16

Mathematics, 19.05.2020 16:16

Mathematics, 19.05.2020 16:16

History, 19.05.2020 16:16

Mathematics, 19.05.2020 16:16


Mathematics, 19.05.2020 16:16



Mathematics, 19.05.2020 16:16


Mathematics, 19.05.2020 16:16




Mathematics, 19.05.2020 16:16