Chemistry, 27.03.2020 18:09 aaronolivera200161
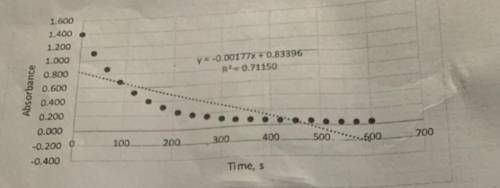
Determine the value of k' from the slope. These graphs were plotted in terms of absorbance, but the rate constant should be in terms of concentration. Beer-Lambert's law and the literature value for the molar absorptivity constant (87,000 M^-1cm^-1 can be used to convert the slope from units of absorbance/s to units of concentration/s. This value is the pseudo rate constant, k'.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
Determine the value of k' from the slope. These graphs were plotted in terms of absorbance, but the...
Questions

Mathematics, 11.03.2021 17:10


Chemistry, 11.03.2021 17:10

Mathematics, 11.03.2021 17:10

Social Studies, 11.03.2021 17:10

Mathematics, 11.03.2021 17:10

Mathematics, 11.03.2021 17:10


English, 11.03.2021 17:10

Biology, 11.03.2021 17:10


English, 11.03.2021 17:10



English, 11.03.2021 17:10

Chemistry, 11.03.2021 17:10

Mathematics, 11.03.2021 17:10

English, 11.03.2021 17:10


Mathematics, 11.03.2021 17:10