
Chemistry, 27.03.2020 16:45 janahiac09
What is the change in freezing point (delta T) of an aqueous solution that is 0.082 molality AlCl3?
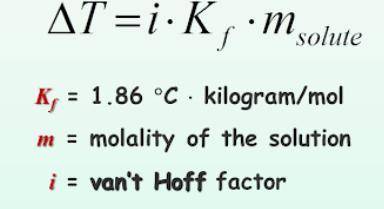

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1


Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
You know the right answer?
What is the change in freezing point (delta T) of an aqueous solution that is 0.082 molality AlCl3?<...
Questions

Mathematics, 08.04.2021 17:10

Social Studies, 08.04.2021 17:10


Mathematics, 08.04.2021 17:10


English, 08.04.2021 17:10




Chemistry, 08.04.2021 17:10

Mathematics, 08.04.2021 17:10

Mathematics, 08.04.2021 17:10

Mathematics, 08.04.2021 17:10









