
Chemistry, 27.03.2020 03:10 drivinghydra
A 0.0900 g sample of household bleach is completely reacted with KI(s). The resulting solution is then titrated with 0.150 M NaS2O3 solution. 0.750 mL of the solution is required to reach the colorless endpoint. What is the mass percent of NaOCl (MM = 74.44 g/mole) in the bleach?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
A 0.0900 g sample of household bleach is completely reacted with KI(s). The resulting solution is th...
Questions


Mathematics, 15.12.2020 19:20



History, 15.12.2020 19:20

Spanish, 15.12.2020 19:20




Mathematics, 15.12.2020 19:20

Mathematics, 15.12.2020 19:20




Mathematics, 15.12.2020 19:20

Mathematics, 15.12.2020 19:20

Mathematics, 15.12.2020 19:20

English, 15.12.2020 19:20


English, 15.12.2020 19:20


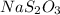 is required for complete neutralization.
is required for complete neutralization.

